Proudly featured in




A HEALTHIER & SAFER TREATMENT
Our Revolutionary Approach
Tivic Health is at the fore of bioelectronic medicine. While this field has relied on implanted devices in the past, Tivic Health is focused on non-invasive approaches, which can be developed with less risk and are more accessible to the people who need them.
KEY elements of the clearup design include:
Clinical Research | ClearUP® Sinus Pain Relief
Our flagship product, ClearUP®, is a groundbreaking, patented handheld device that uses gentle electrical stimulation to target symptoms of sinus and nasal inflammation. ClearUP is designed to relieve congestion, sinus pain, sinus pressure, and sinus headaches caused by allergies, chronic sinus conditions, colds, and the flu. For optimal results, ClearUP works most effectively as part of a regular routine. Continued use maximizes comfort and relief from sinus discomfort.
Clinical Results
Rapid Relief Acheived
of patients experience significant congestion reduction with ClearUP
of patients prefer ClearUP to other allergy treatments
of patients experience pain relief on the first use
of sinus patients try to avoid medication—ClearUp is 100% drug-free
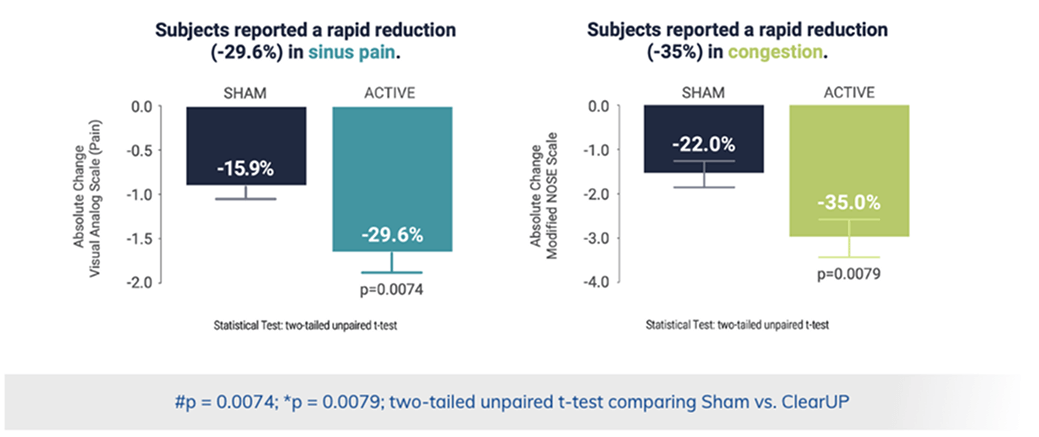
Fast-acting relief within 10 minutes
First clinical trial
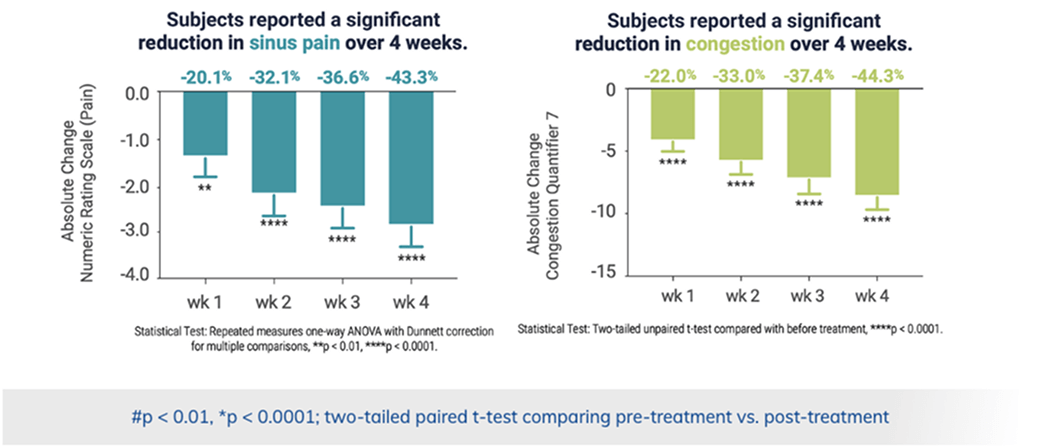
Long-term symptom relief with regular use
Second clinical trial
Subjects used ClearUP at home for 4 weeks and experienced continuous improvement with extended use.
THE PHYSICIAN'S VIEW
Why Physicians Love ClearUP
Subinoy Das, MD
CEO, US Institute for Advanced Sinus Care & Research
Alan Goldsobel, MD
Board Certified Allergist and Adjunct Clinical Professor, Stanford University Medical School
ClearUP Mechanisms of Action: Two Principal Pathways
1. Vasoconstriction
The blood vessels that supply the sinus and nasal mucosa are surrounded by sympathetic nerve fibers.
Electrical stimulation of sympathetic nerve fibers has been shown to promote release of norepinephrine. 1-3
Norepinephrine facilitates smooth muscle contraction around the blood vessels, leading to vasoconstriction. 4
Vasoconstriction of arterioles and venous vessels, in the context of sinonasal inflammation, results in smaller vessel diameter, relieving pressure on the nerves and reducing resistance to air flow.
Over time, repeated vasoconstriction can reduce edema and extravasation of inflammatory immune cells, contributing to reduced symptom severity.
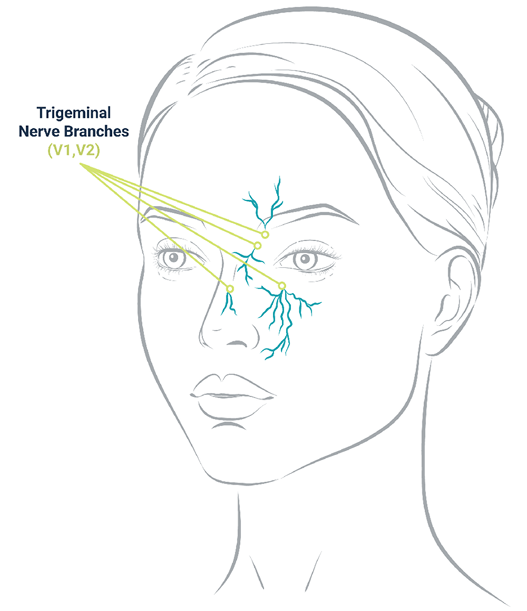
2. Trigeminal Nerve Stimulation
The trigeminal nerve — the ophthalmic nerve (V1) and maxillary nerve (V2) — is responsible for relaying sensory information to the brain.
Electrical microcurrent delivered in the periorbital regions stimulates subcutaneous fibers of these branches.
Neuromodulation of the trigeminal nerve pathway may alleviate sensations of pain and pressure.
Resources
- Mandel, Yossi, et al. “Vasoconstriction by electrical stimulation: new approach to control of non-compressible hemorrhage.” Scientific Reports 3 (2013)
- Franco, O.S., et al. “Effects of different frequencies of transcutaneous electrical nerve stimulation on venous vascular reactivity.” Brazilian Journal of Medical and Biological Research 47.5 (2014): 411-418.
- Malm, L. “Stimulation of sympathetic nerve fibres to the nose in cats.” Acta otolaryngologica 75.2-6 (1973); 519-526.
- Fischer, Laurent, et al. “Adrenergic and non-adrenergic vasoconstrictor mechanisms in the human nasal mucosa.” Rhinology 31.1 (1993): 11-15.
